Emerging Antibody Therapies Targeting B Cell Lymphomas
Since rituximab, novel modalities of highly potent immunotherapies have been investigated and/or marketed to lift the limits on treatment of B cell lymphomas, including NHLs. The past decade has witnessed the approval of plenty of antibody-based, potent therapies of B cell lymphomas, that, along with CAR -Ts, have tremendously changed the therapeutic landscape, including:
1. T Cell Engagers (TCEs)
- 1) Blinatumomab: CD19/CD3 TCE of BiTE format, by Amgen.
- 2) Mosunetuzumab: first ever approved CD20/CD3 TCE, by Roche.
- 3) Epcoritamab: a CD20/CD3 TCE by Abbvie and Genmab; subcutaneously. administrated for better patient compliance and lower CRS liabilities.
- 4) Glofitamab: a 2:1 format CD20/CD3 TCE by Roche; dual-targeting brings T cells in closer proximity for potent target-killing.
2. Antibody-drug conjugates (ADCs)
- 1) Brentuximab vedotin (anti-CD30-MMAE by Seagen/Takeda).
- 2) Polatozumab vedotin (anti-CD79b-MMAE by Genentech).
- 3) Loncastuximab tesirine (anti-CD19-PBD by ADC/Sobi).
However, the clinical effectiveness of approved ones has been impaired by inadequate treatment efficacy, intolerable toxicities, and the development of treatment resistance.
Fortunately, novel antibody therapeutics inspired by unmet medical needs are emerging that, either at early or POC stages or late stage of clinical tests, seem promising for combatting limitations currently seen in clinical practices, as exemplified below.
1. IGM-2323 (Imvotamab) by IGM Biosciences
This TCE of IgM isotype targets CD20 and CD3. With 10-valency units for CD20, it’s designed to bind to CD20 with more power (avidity). Preclinical research demonstrates that it may have advantages over IgG bispecific antibodies, therefore more likely to overcome CD20 low abundance or loss-induced drug resistance, and achieve deeper depletion than currently approved antibody therapies.
Data generated from its Phase 1 clinical trials provide evidence that Imvotamab exhibits a favorable safety and tolerability profile with encouraging activity in refractory or relapsed NHL patients.
2. Odronextamab by Regeneron
This CD20/CD3 TCE is a devised format via the introduction of local isotype chimera of IgG1/3, and promises to be of native format with improved PK property.
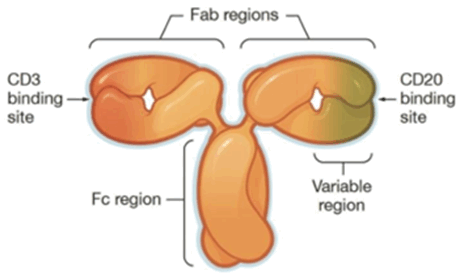
Figure 1. Structure of Odronextamab
Odronextamab has reported new data from its pivotal Phase II study, and is now under FDA priority review.
If approved, odronextamab would be the first and only bispecific antibody approved in both FL and DLBCL – the two most common subtypes of NHL.
3. GB261 (by Genor)
This CD20/CD3 TCE is of ultra-low affinity to bind CD3 and, RARELY, keeps Fc functions (ADCC and CDC). By its designed MOAs, Fc-mediated cytolysis and T cell-mediated killing dominates in according to the change of T/B cell ratios from initial stage of treatment towards late stage.
GB261 significantly inhibited rituximab-resistant cancer cell proliferation and induced but less cytokine release, and, now in clinical trial, promises to be a highly potent TCE for B cell malignancies.
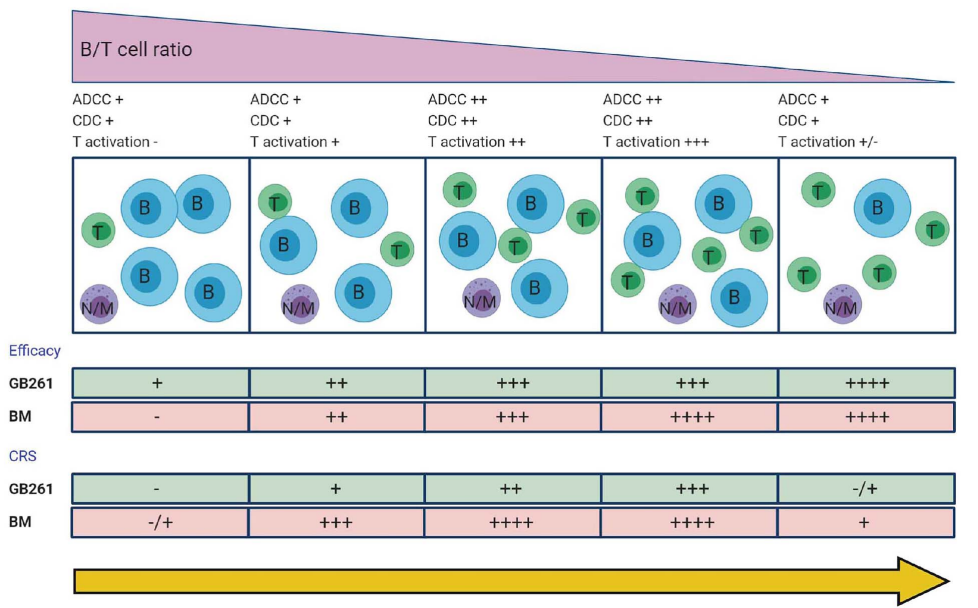
Figure 2. Efficacy of GB261 on CD20+, CD3+ lymphocytes in Cynomolgus monkeys
4. CX-2029 (by CytomX)
This probody drug conjugates directed against CD71 that contains MMAE are conditionally activated, masked ADC, whose activity would be restored by tumor-associated proteases, thereby restricting target engagement to the tumor, creating a therapeutic window for this previously undruggable target.
Now under phase 1/2 evaluation in patients with some solid tumors and DLBCL,
This prodrug showed clinical activity at tolerable doses.
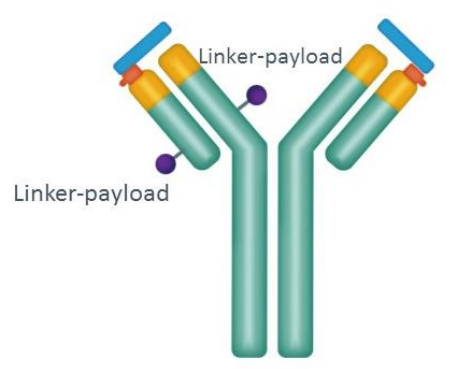
Figure 3. Structure of CX-2029 drug conjugates
5. CH2-aIgM-MMAE (by Technical University of Darmstadt)
When targeting such antigens as mentioned above, while failing to cover all target disease cells, it’s hard to distinguish cancerous B cells from their non-malignant counterparts, leading to on-target toxicities.
An amazing work on CH2-aIgM-MMAE, a human IgM-targeting prodrug ADC, provided a novel mechanism for NHL treatment.
In this excellent POC research:
- 1) Human IgM (constant domain), rather than any CD markers, was targeted for clonal B cell lymphoma eradication. Immunization of chicken instead of mammals, was used to get desired immune response.
- 2) Chicken-derived aIgM was fused to and masked by the epitope-holding IgM domain CH2 via a dual tumor-protease cleavable linker.
- 3) The prodrug was ultimately equipped with MMAE.
- 4) Binding capacity of the prodrug was restored upon conditional protease-mediated demasking which consequently enabled target-dependent antibody internalization and subsequent induction of apoptosis in malignant B cells.
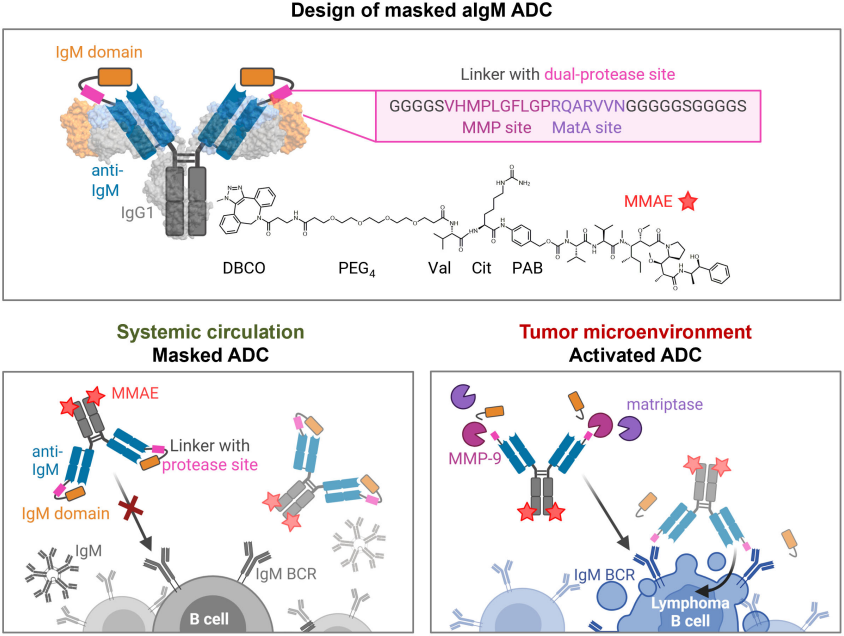
Figure 4. Design and mode of action of masked aIgM ADC.
Limitations on clinical practice are being updated as its boundary are pushed. So are the therapeutic approaches for them. As they are evolving, these approaches will definitely further enhance the chance of cure for B cell malignancies.
Reference
1. Hart, Kevin C., et al. "High valency of IGM-2323, a CD20xCD3 IgM bispecific T cell engager, displaces rituximab binding and induces potent B lymphoma cell killing." Cancer Research. Vol. 82. No. 12. 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA 19106-4404 USA: AMER ASSOC CANCER RESEARCH, 2022.
2. Cai, Wenyan, et al. "Biological activity validation of a computationally designed Rituximab/CD3 T cell engager targeting CD20+ cancers with multiple mechanisms of action." Antibody Therapeutics 4.4 (2021): 228-241.
3. Johnson, Melissa, et al. "P01. 06 CX-2029, a probody drug conjugate targeting CD71 in patients with selected tumor types: PROCLAIM-CX-2029 dose expansion phase." Journal of Thoracic Oncology 16.3 (2021): S238.
4. Johnson, Melissa, et al. "Phase I, first-in-human study of the probody therapeutic CX-2029 in adults with advanced solid tumor malignancies." Clinical Cancer Research 27.16 (2021): 4521-4530.
5. Schoenfeld, Katrin, et al. "Conditional activation of an anti-IgM antibody-drug conjugate for precise B cell lymphoma targeting." Frontiers in Immunology 14 (2023): 1258700.
Detai Bioscience, Inc. is leveraging its single B platform to unlock next-generation antibody discovery generated by rare species. We are working on cases of horse, swine, human (PBMCs), and more to come. Contact us to have rare species-generated antibody custom-made for you.







