Mannose in Targeted Immunotherapeutic Endeavors
Mannose on the surface of many common pathogens serves as a "danger signal" that, not found on healthy human cells and tissues, activates lectin activation pathway of complement system to "bomb" pathogen invaders.
In addition to complements, macrophages are able to sense the invasion as well. When capturing mannose through receptor, the macrophage turns itself from a bored "garbage collector" to a "professional eater" or an "angry killer" against microbial attacks.
Mannose glycosylation is also found to be a “hallmark” in cancer cells.
The mannose characteristics has been evaluated for the potential in targeted and broad-spectrum microorganism inhibition and cancer diagnosis or therapy. Some of research endeavors are listed and discussed below.
- High mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting.
The aptamers tested exhibited high affinity toward spike protein of SARS-CoV-2 & envelope protein GP120 of HIV and a certain inhibition effect. Meanwhile, they specifically recognized cancer cells over normal cells.
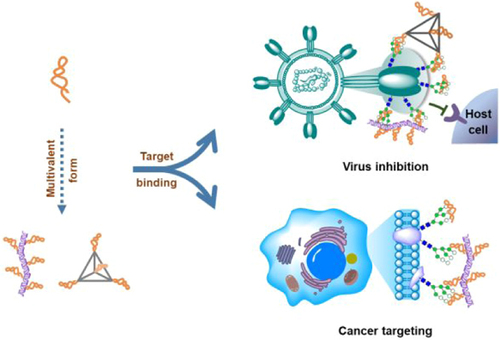
Figure 1. Mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting
- Mannose modification for targeted photothermal therapy
Photothermal therapy is promising to conquer cancer resistance. To arm it with cancer selectivity, nanoparticle used in the therapy was equipped with mannose as targeting unit. Data indicated the potential application of photothermal therapy of tumor for accurate diagnosis.
- Mannose-modification for targeting TAMs
Tumor-associated macrophages (TAMs) are a class of immune cells that amount to substantial tumor mass. They contribute significantly to immunosuppressive TME and the resistance to chemotherapy drugs and nano-medicine, and therefore are rising as targets in cancer treatment, via either polarization into M1-like phenotype or depletion.
A study with mannose-modified nanocarriers displayed TAM-targeting delivery in solid tumors.
If loaded with proper cargo, these carriers could be used for prognosis or therapeutic purposes.
Here comes the one that’s loaded with therapeutic cargo. In order to endow chemotherapy with tumor precision, a research group developed mannose-modified liposomes co-loaded with ZOL and DOX, and offered results indicating remarkably enhanced anticancer effects via depleting TAMs.
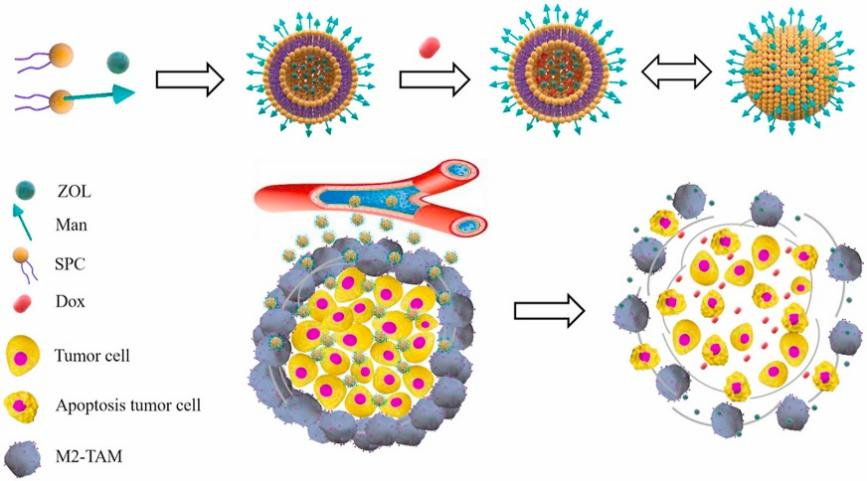
Figure 2. Man-LP@ZOL/DOX developed by Cai. could deplete M2-tumor-associated macrophages to enhance anti-tumor effect of doxorubicin on TNBC
- Lectibody that targets mannose for pan-tumor treatment.
As discussed, cancer-related glycomics, including mannose glycosylation, is found to be a “hallmark” in cancer cells, and now is a treasure waiting to be mined for pan-tumor drug development.
AvFc, a lectibody constructed by fusing glycan-binding lectin to human IgG1, was found to significantly restrict tumor growth in models, which provides POC evidence that mannose glycans in the glycocalyx of cancer cells can be a pan-tumor, druggable target.
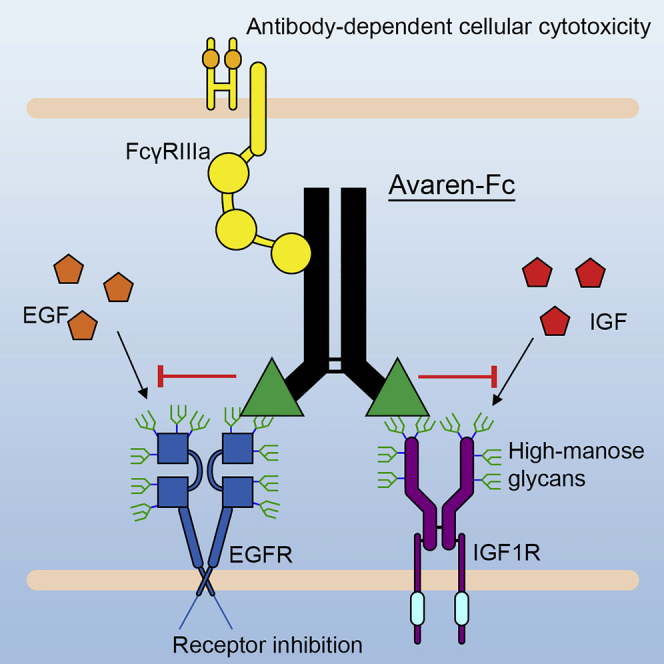
Figure 3. AvFc, a lectibody that targets mannose for pan-tumor treatment
Mannose is rarely used for glycolysis in the normal tissues of mammals, which makes it a “sweet” option in cancer treatment as in pathogen fighting, so sweet as to make “one-therapeutic-fit-all” therapies expected.
Reference
1. Bangarh, Rashmi, et al. "Aberrant protein glycosylation: Implications on diagnosis and Immunotherapy." Biotechnology Advances (2023): 108149.
2. Li, Wei, et al. "High mannose-specific aptamers for broad-spectrum virus inhibition and cancer targeting." CCS Chemistry 5.2 (2023): 497-509.
3. Li, Jiaxu, et al. "Mannose modified zwitterionic polyester-conjugated second near-infrared organic fluorophore for targeted photothermal therapy." Biomaterials Science 9.13 (2021): 4648-4661.
4. Fernández-Mariño, Iago, et al. "Mannose-modified hyaluronic acid nanocapsules for the targeting of tumor-associated macrophages." Drug Delivery and Translational Research 13.7 (2023): 1896-1911.
5. Wendong, Yao, et al. "Mannose modified co-loaded zoledronic liposomes deplete M2-tumor-associated macrophages to enhance anti-tumor effect of doxorubicin on TNBC." Journal of Drug Delivery Science and Technology 74 (2022): 103551.
6. Oh, Young Jun, et al. "Antitumor activity of a lectibody targeting cancer-associated high-mannose glycans." Molecular Therapy 30.4 (2022): 1523-1535.
With its unparalleled single B antibody discovery approach and unique antigen design strategy, Detai Bioscience, Inc. is overcoming the low immunogenicity of vitamins, toxins, and glycans, and is discovering high-quality antibodies against these haptens to enable alternative cancer therapies and upgraded in vitro diagnosis (IVD). Contact us for details of how we can help.






