Molecular Construction of T Cell Engagers: Formats Matter
T cell engagers (TCEs) are artificial, multi-specific therapeutic antibodies for cancer treatment, engineered to redirect the immune system's T cells to recognize and kill cancer cells. They are designed to bind to a target antigen expressed on a cancer cell and to a trigger molecule on T cells, generally CD3. Formats of TCEs, as to other multi-specifics, matter a lot for their manufacturability and PK/ADA property as well as efficacy. Different formats have been developed, each with ads and drawbacks. Representatives of some significantly developed TCEs, either preclinically or clinically, are illustrated in this review.
IgG-like TCEs
IgG-like TCEs, due to limited mutations introduced, retain many of the favorable traits of conventional IgGs.
Unlike the generation of Catumaxomab (Epcam/CD3 TCE), specific and smart strategies to encourage desirable LC/HC and/or HC/HC pairing and to facilitate purification are essentially required and actually utilized to successfully construct the new-generation, premium format, as exemplified below.
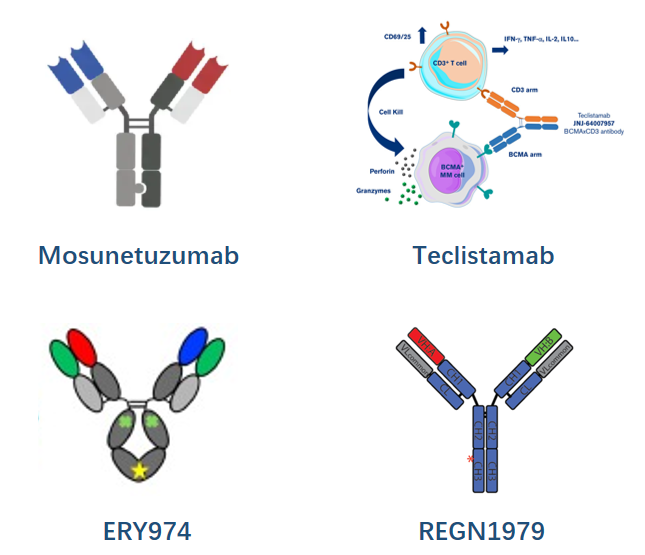
- Mosunetuzumab by Genentech
Mosunetuzumab is constructed with knobs-into-holes technology for desired HC pairing while in vitro annealing for HC/LC pairing, and was approved for the treatment of R/R follicular lymphoma.
- Teclistamab by JNJ
It's a BCMA-targeting DuoBody that has been marketed for Relapsed/Refractory Multiple Myeloma (R/R MM) treatment
- DuoBody
A controlled Fab-arm exchange technology developed by Genmab, enables efficient generation of bsAbs with normal IgG structures.
- Epcoritamab
It's another DuoBody co-developed by AbbVie and Genmab, and was recently approved for Relapsed/Refractory diffuse large B-cell lymphoma (R/R DLBCL) treatment.
- ERY974 by Chugai (ART-Ig)
ERY974 is an anti–GPC3/CD3 TCE for treatment of solid tumors under development preclinically. Its construction is achieved through introduction of electrostatic steering mutations in the CH3 domain to drive heterodimerization of the heavy chains and utilization of a common LC to avoid LC mispairing.
- REGN1979 by Regeneron
This CD20/CD3 TCE, now in clinical trials for B-NHL, is a devised format via the introduction of local isotype chimera of IgG1/G3 in CH3 in addition to the utilization of a common LC.
This format promises to be a more native one since it avoids Fc-Fc interface engineering, therefore further reducing PK/ADA and manufacturability risks.
Based on this technology, a few more TCEs for solid tumors, including REGN4018, REGN4336(PSMA/CD3 for mCRPC), are under clinical evaluations.
Fugure 1. Fragment-based TCEs
While IgG-like formats hold traits of native IgG favorable for manufacturability and PK/ADA property of antibody drugs, fragment-based T cell engagers (TCEs) have been developed anyway, although immunogenicity and instability result due to unnatural mutations or fragments introduced, for achieving unique medical purposes, or due to the unavailability of constructing technology, or commercial limitations.
Fragment-based TCEs are generally
- Simple structures comprised of linked scFvs, scFabs or sVDs, et al.
- Lacking half-life extending (HLE) part.
- Challenging to produce, prone to aggregate/fragment.
- Of small size with short half-life.
Representative TCE of this kind is blinatumomab(CD19/CD3 BiTE, two linked, independent scFvs) by Amgen approved for treatment of BCP-ALL and more later on.
In order to prolong half-life and allow less frequent dosing, BiTE molecules have been genetically fused to an Fc domain, resulting in a series of HLE BiTE molecules (MW around106 kD) in Amgen.
AMG757, one of HLE BiTEs by Amgen targeting DLL3, is under investigation for SCLC treatment.
In a similar fashion, MacroGenics DART platform designs therapeutic molecules that combine two independent Fabs in a diabody-like structure with or without Fc domain tailored to have a long or short half-life, respectively.
MGD024, a CD123 x CD3 Dart TCE for patients with R/R CD123(+) hematologic malignancies, has entered clinical investigation.
Meanwhile, with its TriTAC and ProTriTAC(prodrug) TCE platforms, Harpoon has created fragment-based TCEs that incorporate single domain antibodies and use albumin binding for half-life extension.
Furthermore, tebentafusp-tebn(KIMMTRAK), the first and only approved TCE for solid tumor treatment (mUM) developed by Immunocore, is composed of a TCRm and a CD3-targeting scFv. It has an acceptable PK profile due to its increased size (77KD).
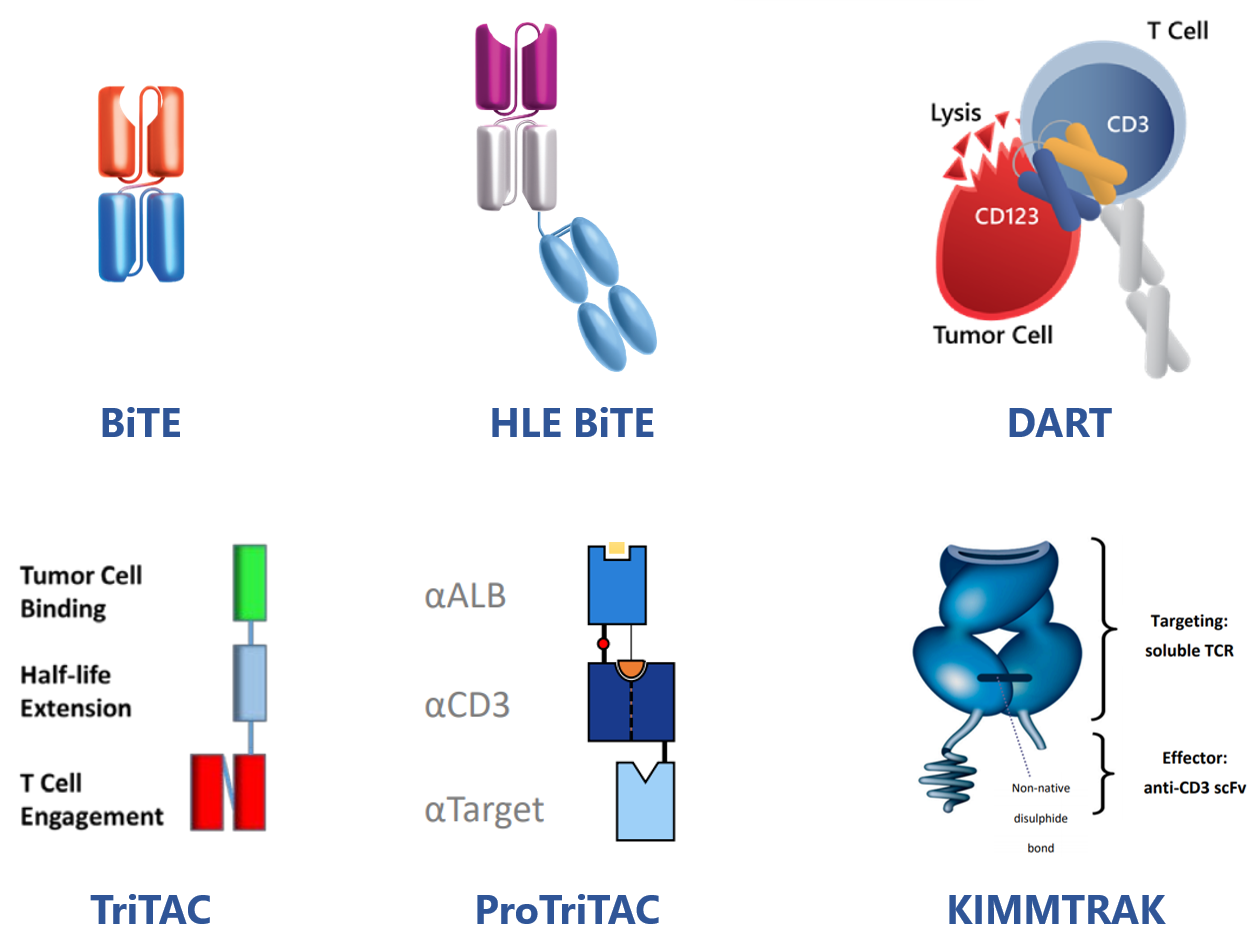
Figure 2. TCEs Other Than Above Discussed
In order to acquire improved efficacy/safety profile or to further enhance clinical significance, numerous differentiated formats in addition to fragment-based and IgG-like ones have been designed and tested, some of which are discussed below.
- EMB-07.
This TCE (ROR1/CD3) is an appended-IgG of symmetrical structure (2:2) designed with FIT-Ig platform of EpimAb for solid tumors, and is now in clinical evaluation.
Appended-IgGs are conventional IgGs appended with scFv, Fab, or nanobody, et al. They have half-life comparable to parental ones. However, non-natural structures might give rise to increased aggregation, instability and ADA risk. FIT-Ig platform generates bispecific antibodies without introducing mutations or linkers, favoring their manufacturability and stability.
EMB-06, another FIT-Ig generated TCE targeting BCMA, is also under clinical evaluation.
- BA1202.
This CEA/CD3 symmetrical (2:2) TCE by Boan Biotech is of a butterfly-shaped antibody structure: Fab ends bind bivalently to CEA while LC-NT linked bivalent scFvs bind to CD3 monovalently (possibly due to altered spatial constructure and impaired affinity compared to Fc-hinge linked one), and has entered clinical trial.
- Cibisatamab (RG7802).
It's a 2:1 format CEA/CD3 TCE by adding a second CEA-binding Fab to asymmetrical 1:1 format to enable a "low affinity but high avidity" binding to disease targets. It presumably will achieve optimized efficacy and safety by decreasing "on-target-off-tumor toxicity", and is now under development by Roche in clinical trials.
- CX-904.
Designed by Cytomx for precision targeting, this clinical-stage 2:2 EGFR/CD3 TCE prodrug is conditionally activated in tumor microenvironment upon specific proteolysis.
- Imvotamab (IGM-2323).
This TCE of IgM isotype by IGM targets CD20 and CD3. With 10-valency units for CD20, it’s proposed to bind to CD20 with more power (avidity) than IgG format, therefore overcoming target decrease-induced drug resistance, and achieving deeper depletion than currently approved antibody therapies.
More IgM TCE drugs from this company include IGM-2644 (CD38/CD3) and IGM-2537 (CD123/CD3).
- TNB-486.
This CD19-targeting TCE’s asymmetrical format by Teneobio falls kind of between the fragment-based and the IgG-like. It’s comprised of a CD3-binding half IgG and a CD19-binding HcAb, and is now under clinical evaluation for relapsed/refractory (R/R) B-lymphoma.
- YBODY.
Similar with the format of TNB-486, YBODY platform by YZYBio designs TCE that is composed of disease target-binding half IgG and CD3-binding scFv.
A few YBODY TCEs are now in clinical trials, such as M802 (HER2/CD3).
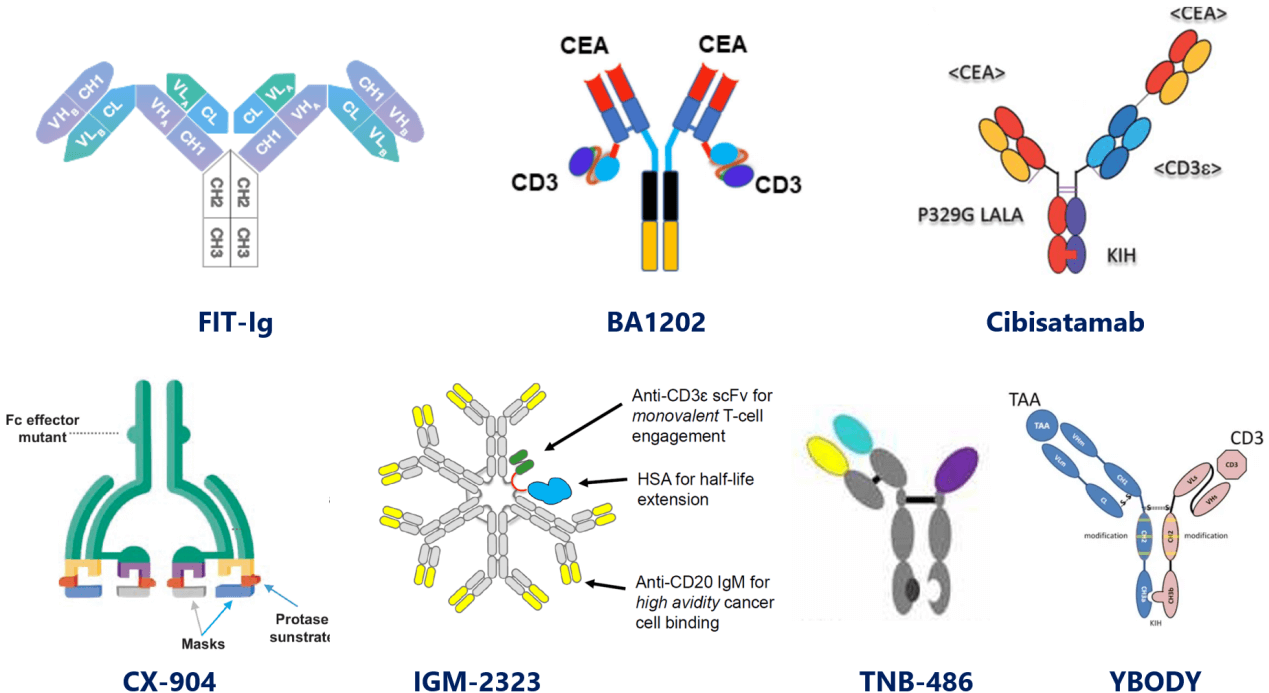
Figure3. IgG-like TCEs in differentiated formats
Reference
1.Giese, Glen, et al. "Bispecific antibody process development: Assembly and purification of knob and hole bispecific antibodies." Biotechnology progress 34.2 (2018): 397-404.
2.Pillarisetti, Kodandaram, et al. "Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma." Blood advances 4.18 (2020): 4538-4549.
3.Labrijn, Aran F., et al. "Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange." Proceedings of the National Academy of Sciences 110.13 (2013): 5145-5150.
4.Zhang, Jimin, et al. "Pharmacodynamic activity of epcoritamab (GEN3013; CD3xCD20) as monotherapy is maintained in combination with standard of care therapies in patients with diffuse large B-cell lymphoma." Cancer Research 83.7_Supplement (2023): 3248-3248.
5.Shiraiwa, Hirotake, et al. "Engineering a bispecific antibody with a common light chain: Identification and optimization of an anti-CD3 epsilon and anti-GPC3 bispecific antibody, ERY974." Methods 154 (2019): 10-20.
6.Ishiguro, Takahiro, et al. "An anti–glypican 3/CD3 bispecific T cell–redirecting antibody for treatment of solid tumors." Science translational medicine 9.410 (2017): eaal4291.
7.Smith, Eric J., et al. "A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys." Scientific reports 5.1 (2015): 17943.
8.Leung, Shuk on Annie, and Panagiotis A. Konstantinopoulos. "Advances in the treatment of platinum resistant epithelial ovarian cancer: an update on standard and experimental therapies." Expert Opinion on Investigational Drugs 30.7 (2021): 695-707.
9.Kelly, William Kevin, et al. "A phase 1/2 study of REGN4336, a PSMAxCD3 bispecific antibody, alone and in combination with cemiplimab in patients with metastatic castration-resistant prostate cancer." (2022): TPS5105-TPS5105.
10.Portell, Craig A., Candice M. Wenzell, and Anjali S. Advani. "Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia." Clinical pharmacology: advances and applications 5.sup1 (2013): 5-11.
11.Jen, Emily Y., et al. "FDA approval: blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease." Clinical Cancer Research 25.2 (2019): 473-477.
12.Giffin, Michael J., et al. "AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer." Clinical Cancer Research 27.5 (2021): 1526-1537.
13.Huang, Ling, et al. "Multispecific, Multivalent Antibody‐Based Molecules Engineered on the DART® and TRIDENTTM Platforms." Current Protocols in Immunology 129.1 (2020): e95.
14.Winer, Eric S., et al. "A Phase 1, First-in-Human, Dose-Escalation Study of MGD024, a CD123 x CD3 Bispecific Dart® Molecule, in Patients with Relapsed or Refractory CD123-Positive (+) Hematologic Malignancies." Blood 140.Supplement 1 (2022): 11753-11754.
15.Hua, Gwen, Daniel Carlson, and Jacqueline R. Starr. "Tebentafusp-tebn: A Novel Bispecific T-Cell Engager for Metastatic Uveal Melanoma." Journal of the Advanced Practitioner in Oncology 13.7 (2022): 717.
16.Bacac, Marina, et al. "A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors." Clinical Cancer Research 22.13 (2016): 3286-3297.
17.Boustany, Leila M., et al. "A Probody T Cell–engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity." Cancer Research 82.22 (2022): 4288-4298.
18.Hart, Kevin C., et al. "High valency of IGM-2323, a CD20xCD3 IgM bispecific T cell engager, displaces rituximab binding and induces potent B lymphoma cell killing." Cancer Research. Vol. 82. No. 12. 615 CHESTNUT ST, 17TH FLOOR, PHILADELPHIA, PA 19106-4404 USA: AMER ASSOC CANCER RESEARCH, 2022.
19.Malik-Chaudhry, Harbani K., et al. "TNB-486 induces potent tumor cell cytotoxicity coupled with low cytokine release in preclinical models of B-NHL." MAbs. Vol. 13. No. 1. Taylor & Francis, 2021.
20.Hou, Jing-Zhou, et al. "Interim Results of the Phase 1 Study of Tnb-486, a Novel CD19xCD3 T-Cell Engager, in Patients with Relapsed/Refractory (R/R) B-NHL." Blood 140.Supplement 1 (2022): 1474-1475.
21.Jacobs, R., R. Nair, and S. G. Cho. "High complete response rate with TNB-486 in relapsed/refractory follicular lymphoma: Interim results from an ongoing Phase I study." European Hematology Association (EHA) (2023): 8-11.
22.Yu, Shengnan, et al. "A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells." Journal of Experimental & Clinical Cancer Research 38 (2019): 1-16.
DetaiBio provides high-quality design & expression services for mono- and multi-specific antibodies, including scFv, Fab, (Fab')2, VHH, chimeric antibody, bispecific antibody of variable formats, Fc fusion protein, full-length IgG and IgM. The services cover fast, small amount production, stable cell line construction and industry-level antibody production, and more. Please contact DetaiBio for more details.






